Many years ago I read that if the Earth were the size of a cue ball, it would be as smooth and round as a cue ball.
That was startling.
And I questioned it. Along the lines of, well, Mauna Loa rises about 5 miles off the seafloor. And the Mariana Trench goes down about 6 miles. So, could you feel Mauna Loa as a bump or the Mariana Trench as a groove?
OK, a pool ball is a bit over 2 inches in diameter and the Earth is about 8000 miles through the center. So, a mile would be about 1/4000th of an inch on the Earth-ball. 5 or 6 miles makes these two extremes, Mauna Loa and the Mariana Trench, about 1/1000th of an inch on the Earth-ball.
To get a line on how big 1/1000th of an inch is, I pulled out my Autologic printer’s loop and found that the steps leading up to the Lincoln Memorial on the back of a $5 bill are about 5/1000ths of an inch apart. The horizontal lines that are the sky in the Memorial picture are double that at about 1/100th of an inch apart. To my eyes the sky looks like lines when looked at closely. The steps look gray.
Another estimate of now much 1/1000ths of an inch is: Thin paper runs 2 or 3 1000ths of an inch in thickness. Normal paper is in the 4 to 8 range.
The bump at the edge of a piece of paper is easy to feel.
But Mauna Loa would be pretty small – not a long cliff like the edge of a piece of paper. Just one bump. And the bump wouldn’t be a lot wider than it is high – triple, say. Maybe that Earth-ball might feel vaguely blemished to a blind movie hero, but it sure would feel pretty smooth to people who work with their hands.
That isn’t the end of things.
I wondered about that Earth-ball.
For instance, what would it feel like?
Consider that the Earth is covered by ocean a couple of miles deep, on average. Would the Earth-ball feel sorta like it just came in from the cold – steamed up? That’d be about the most that could be detected of the oceans.
If the atmosphere were solid, it would be a paper-thin shell around the whole Earth-ball.
And speaking of paper-thin shells around the Earth-ball, that’s about what the Earth’s crust would be.
Which gets to the fun point:
What if set an Earth-ball down on a pool table? What would happen?
Tick, tick, tick.
Best I can figure is it would expand a bit and burn its way through to the floor, splatting out as a lump of molten metal and lava. The Earth, after all, appears to be like a balloon with the rubber part being cool rock and the air inside replaced by soft, hot rock, or molten metal, or iron that’s so compressed it’s solid.
Here’s a to-scale picture I found at http://einstein.byu.edu/~masong/HTMstuff/textbookpdf/C30.pdf:
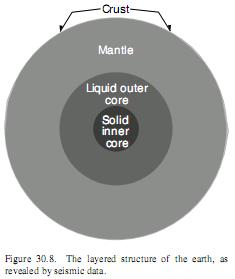
The mantle is hot rock. The core, liquid and solid, is iron. Or so it’s believed. There is no question that it gets hot going down in the crust. Deep mines get real hot – unlivably hot.
Side note: The density of the Earth is almost identical to the density of Radium – at room temperature and pressure. Which isn’t like 2000 miles under the Earth. But, hey, who’s counting?
Anyway, this whole line of thought raises the question:
What keeps the outer shell of the Earth cool?
